• Periodic table of elements
• When an atom has no charge
• Energy levels and principal quantum number (n)
• Energy levels and secondary quantum number
• Orbitals and magnetic quantum number (m)
• Electronics configurations
• How energy levels vary
• Electronics configuration at fundamental state
• Interaction between atoms
• Natural radioactivity
• Natural radioactivity transformations
• Nuclear reactions
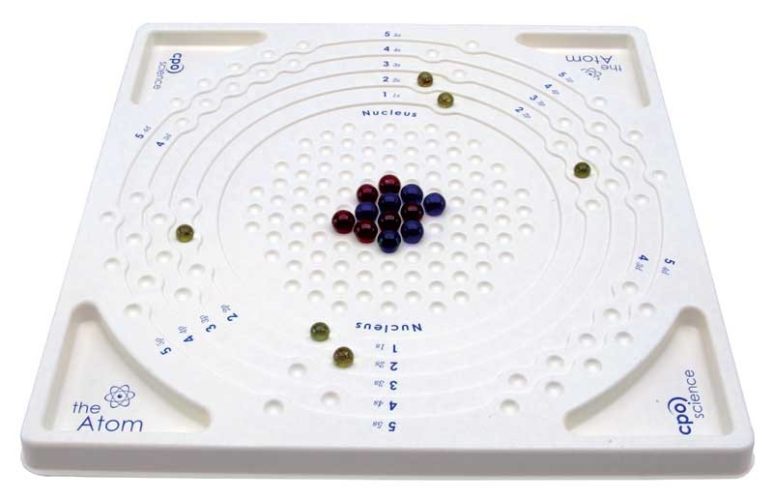
1 Atom model (table)
48 Electrons (yellow spheres)
57 Protons (red or green spheres)
57 Neutrons (black spheres)
48 Cards regarding photons absorption
48 Cards regarding nuclear reactions
2 Periodic table of elements